Model-Free Analysis
Model-free analysis allows to find the activation energy E(α) of the reaction process and b(α) = A(α)·f(α) without assumption of a kinetic model for the process and also without knowledge of the reaction type. Denoted as α is the degree of reaction also called conversion, A(α) is the pre-exponential factor and f(α) describes the reaction type. For model-free analysis, at least two measurements with different temperature programs are required, three or more measurements are recommended.
The first assumption for model-free analysis: the reaction can be always described by only one kinetic equation:
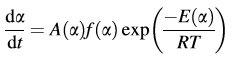
The second assumption for model-free analysis: the reaction rate at a constant value of conversion is only a function of temperature.
It should be noted that the pre-exponential factor A(α) can be found by model-free analysis only with assumption of known function f(α), where a 1st order reaction with f(α) = 1- α is assumed.
For the calculation of the master plot it is assumed that A(α) is constant and f(α)/f(0.5) is calculated as f(α)/f(0.5) = b(α)/b(0.5)with b(α) = A(α)·f(α).
In model-free analysis, the thermoanalytical signal is equal to the reaction rate (1), multiplied by the total effect of reaction: total enthalpy for DSC or total mass loss for TG.
Model-Free Analysis for Incomplete Data
If the measured curves are incomplete, and the final point does not correspond to 100% of conversion, then the total effect (e.g. total mass loss for TG or total peak area for DSC) can be typed manually. Details can be found in the page Incomplete Data .
For the incomplete data model-free analysis provides correct results only for the conversion points measured in all curves.
However, Numerical method in Kinetics Neo calculates activation energy for those conversion values, where more than one measured curve is present.
See more details in our How To guide How to calculate Conversion for Incomplete Measured Data.
Model-Free Methods
Overview of Model-Free Analysis Methods under Project:

For details, see chapters: