Model-Free: Isothermal Arrhenius
The model-free analysis according to Isothermal Arrhenius is an isoconversional method only for time-to-event values (sometimes also called time-to-failure, see ASTM E1877: Calculating Thermal Endurance of Materials from Thermogravimetric Decomposition Data). At least two pairs of data (isothermal temperature; time-to-event) are required from measurements at different isothermal temperatures, four pairs of data are recommended.
Isothermal Arrhenius is a special case of ASTM E2070 D.
For each isothermal measurement, only the time-to-event value is used; at this time point the material properties change significantly. Such data are for example obtained from OIT (Oxidation Induction Time) tests; the time-to-event is in this case the oxidation induction time which is the time until the start of oxidation of a sample exposed to air.
From each measurement, the pair of data (isothermal temperature; time-to-event) has to be typed into the table Time to Event. New lines are created using Add Point:
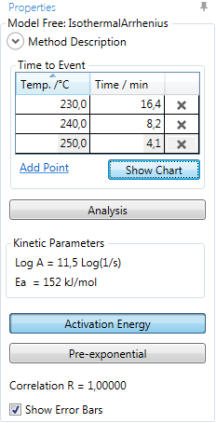
Show Chart displays the (isothermal temperature; time-to-event) data in a chart:
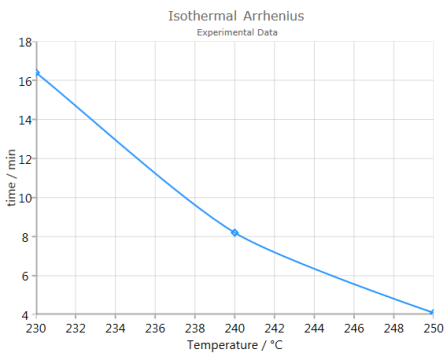
Analysis
Analysis shows a graphic of Log(time-to-event) vs. inverse temperatures of the data. A linear fit curve is furthermore depicted:
Activation Energy and Pre-exponential results are found from the slope and intersect of the linear fit. For calculation of the Pre-exponential, a first order reaction is assumed and furthermore that the event occurs at 5% conversion. Predictions based on Isothermal Arrhenius are therefore only valid for 5% conversion.
The calculated Correlation R is explained in section Statistics.
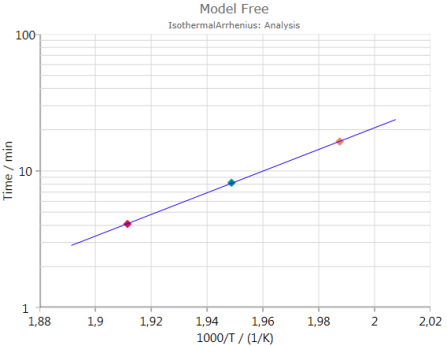
Activation Energy
Pressing
![]()
shows the calculated activation energy including error bar – if the checkbox Show Error Bars is activated:
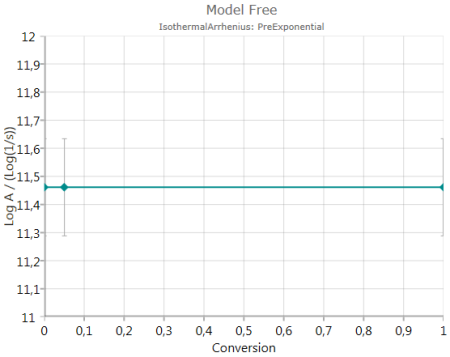
Pre-Exponential Factor
Pressing
![]()
shows the calculated pre-exponential including error bar – if activated.
The chart presents decimal logarithm of pre-exponential factor.
Advantages
Advantages of this method:
-
-No entire measurement required, just the time to event
Disadvantages
Disadvantages of this method:
- only for one-step reactions. For complex reactions, the points are not on a straight line
- only for a set of several isothermal measurements
- only one point is evaluated, all other information is not considered