Model-Free: Dynamic Arrhenius
The model-free analysis according to Dynamic Arrhenius is an isoconversional, integral Ozawa method only for the temperature-to-event values, also called failure temperature values. At least two pairs of data (heating rate; temperature-of-event) are required from measurements at different heating rates, four pairs of data are recommended.
Dynamic Arrhenius is calculated according to ASTM E1641. Results are referred as failure temperatures in ASTM E1877.
For each dynamic measurement, only the temperature-of-event value is used; at this temperature, the material properties change significantly upon heating. A typical example is the dynamic OIT (Oxidation Induction Temperature) also called OOT (Oxidation Onset Temperature): the onset temperature of oxidation for a material heated in air atmosphere.
From each measurement, the pair of data (heating rate; temperature-of-event) has to be typed into the table Temperature of Event. New lines are created using Add Point:
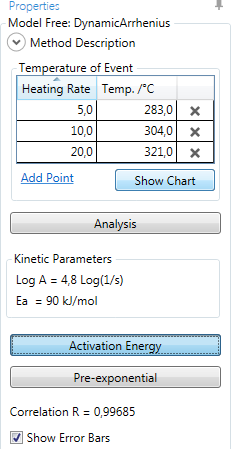
Show Chart displays the (heating rate; temperature-of-event) data in a chart:
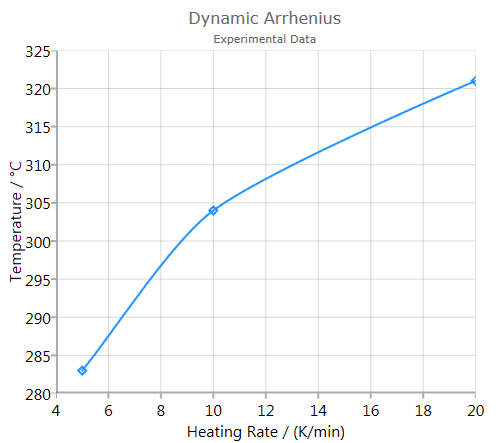
Analysis
Analysis shows a graphic of Log(heating rate) vs. inverse temperature-of-event. A linear fit curve is furthermore depicted:
Activation Energy and Pre-exponential results are found from the slope and intersect of the linear fit. For calculation of the Pre-exponential, a first order reaction is assumed and furthermore that the event occurs at 5% conversion. Predictions based on Dynamic Arrhenius are therefore only valid for 5% conversion.
The calculated Correlation R is explained in section Statistics.
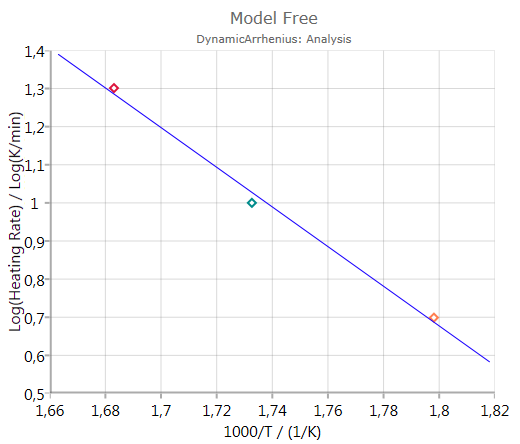
Activation Energy
Pressing
![]()
shows the calculated activation energy including error bar – if the checkbox Show Error Bars is activated:
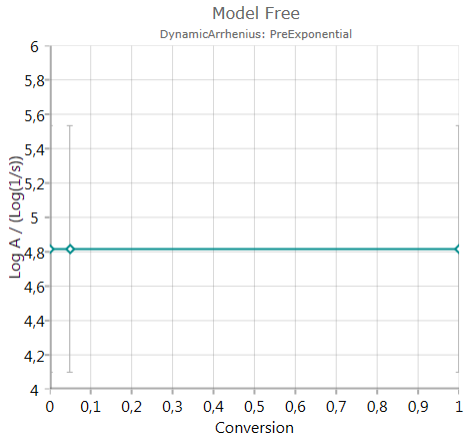
Pre-Exponential Factor
Pressing
![]()
shows the calculated pre-exponential including error bar – if activated.
The chart presents decimal logarithm of pre-exponential factor.
Advantages
Advantages of this method:
- No entire measurement required, just the temperature-of-event
Disadvantages
Disadvantages of this method:
- only for one-step reactions. For complex reactions, the points are not on a straight line
- only for a set of several dynamic measurements
- only one point is evaluated, all other information is not considered