Model-Based Analysis
For model-based analysis, at least two measurements with different temperature programs are required, three or more measurements are recommended.
The rate of kinetic process chemical reaction or crystallization depends on the current concentrations of reactants and on temperature. In general, the reaction rate of the simple reaction can be written as the product of two functions: the first f(c) depends on concentrations and the second K(T) depends on temperature.
Reaction rate=A*f(c)*K(T)
Where A is the pre-exponential factor.
Temperature dependence for chemical reactions usually have temperature dependence according to Arrhenius with activation energy E:
K(T)=exp[-E/(RT)]
Crystallization has another temperature dependence K(T,Tm,Tg) depending on the melting temperature Tm and glass transition temperature Tg.
The first assumption for the model-based method
The reaction consists of several elementary reaction steps, and the reaction rate of each step can be described by an own kinetic equation for this step, depending on the concentration of the initial reactant ej, the concentration of product pj, the pre-exponential factor Aj and the activation energy Ej, specific only for this step with number j
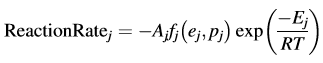
Each step has an own reaction type described by the function fj (ej;pj).
Some examples of such functions are: Second-order reaction has f = e2, Prout–Thompkins reaction with acceleration has f = empn, reaction with one-dimensional diffusion has f = 0.5/p. The number of kinetic equations is equal to the number of the reaction steps, the concentration for each reactant increases for the reaction steps where this reactant is a product, and decreases for the reaction steps, where this reactant is a starting substance.
The second assumption for model-based analysis
All kinetic parameters like activation energy, pre-exponential factor, order of reaction, and reaction type are assumed constant during the reaction progress for every individual reaction step.
The third assumption for model-based analysis
The total thermoanalytical signal is the sum of the signals of the single reaction steps. The effect of each step is calculated as the reaction rate multiplied by the effect of this step (for example enthalpy change or mass loss).
Note
For reactions with Diffusion Control assumed in case of projects of type DSC Curing, and for Crystallization Kinetics, the temperature dependence of the reaction rate is more complicated than the Arrhenius function exp(-Ej/RT).
If reaction rate depends on both temperature and Additional Parameter like Pressure, Intensity of UV light or reactant concentrations, then the common kinetic model can be created.
For more details, see chapters:
Model-Based Analysis: Guideline
Model-Based Analysis: Reaction Types
and also our web site kinetics.netzsch.com, section Equations used in kinetic models.