Reversible Reactions
Support of Reversible Reactions is implemented in Kinetics Neo since version 3.0.
In reversible reactions A⇌B, two chemical reactions occur simultaneously.
The first one is the forward reaction A → B, the second one is the backward reaction is B → A.
In the closed systems the concentrations of A and B are in equilibrium, where the rates of forward reaction and backward reaction are equal.
However, in thermal analysis like DSC or TG, the system is open, and no equilibrium happens. The total reaction rate of measured data is the difference between the forward reaction and backward reaction:
Reaction ratetotal= Reaction rateforward – Reaction ratebackward
Reaction type FnR describe the total reaction rate where both forward and backward reactions are reactions of n-th order:
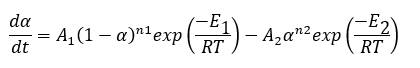
To separate the backward reaction from the forward reaction for analysis, we need several measurements, where for the same temperature total reaction rate is different.
The easiest way for this is to perform the measurement of reversible reaction in reactive atmosphere, where reactive gas has the influence either on forward reaction only or on backward reaction only.
We recommend to use reversible reaction FnR for measurements, where either forward or backward reaction are pressure-dependent.
Example: measurements with different partial pressure of reactive gaseous component for backward reaction in reversing reactions, like decomposition of Calcium carbonate under presence of carbon dioxide.
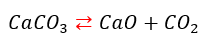
The rate of forward reaction is independent from pressure. The backward reaction has the active gaseous reactant CO2. The rate of backward reaction is higher for higher partial pressure of CO2. The cumulative reaction becomes to be slower:
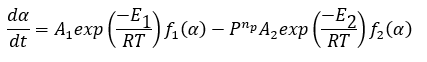
Where P is the partial pressure of carbon dioxide, and np is the pressure parameter.
On our main web site you can find mode information about reversible reactions and practical How To guide How to Analyze Reversible Reaction with Reactive Gaseous Reactant (thermal decomposition of Calcium Carbonate in atmosphere with carbon dioxide).