Model-Based Analysis: Adjusting Arrows
2. Additional Arrow Buttons and Version Information
3. Names of Arrows: Adjustment Arrows Buttons and Their Actions
4. General Figure and Curve Description Used In This Guide
5. Using of the Adjusting Arrow Buttons
5.1  Move current
reaction step to the EARLIER time
Move current
reaction step to the EARLIER time
5.2  DECREASE
Activation Energy and move the step EARLIER
DECREASE
Activation Energy and move the step EARLIER
5.3  INCREASE
contribution of the current step
INCREASE
contribution of the current step
5.4  DECREASE
contribution of the current step
DECREASE
contribution of the current step
5.5  INCREASE
Activation Energy and move the step LATER
INCREASE
Activation Energy and move the step LATER
5.6  MOVE
current reaction step to the LATER time
MOVE
current reaction step to the LATER time
5.7  SPREAD
current reaction step
SPREAD
current reaction step
5.8  COMPRESS
current reaction step
COMPRESS
current reaction step
5.9  SHARPEN #
current reaction step
SHARPEN #
current reaction step
5.10  FLATTEN
current reaction step
FLATTEN
current reaction step
Introduction
In model-based analysis the software searches the optimal kinetic parameters in order to get the best fit for the experimental curves. This optimization task is non-linear for the models with several reaction steps, and result depends on the initial values of parameters. The closer these initial values are to the optimal values the better will be the resulting fit after optimization.
It is not always necessary, but sometimes it is extremely useful and time saved to adjust the simulated curves manually, before optimization, to get them closer to the source measured data. This can be done by using the adjusting arrow buttons.
For example, it is possible manually:
-
to move the simulated curves to the left or to the right,
-
to bring the simulated curves closer to each other or far from each other,
-
to correct the shape of the simulated curves,
-
to correct the contribution of the current step.
2. Additional Arrow Buttons and Version Information
Complete set of 10 adjusting arrow buttons including 6 new buttons is available since Kinetics Neo version 2.4. In the earlier versions only 4 arrow buttons (EARLIER, LATER, INCREASE, DECREASE) are present.
In Model Based Analysis there are 8 standard arrow buttons for the manual adjusting of selected step. These buttons are always visible.
The number of adjusting arrows can be increased to 10 by selecting the checkbox in Settings - Show two additional arrows for adjusting step by Activation Energy in Model-based Analysis.
In this case the full set of 10 adjusting arrow buttons including 2 additional arrow buttons with red border will be shown.
3. Names of Arrows: Adjustment Arrows Buttons and Their Actions
1.  MOVE current reaction step to the EARLIER time
MOVE current reaction step to the EARLIER time
2.  DECREASE Activation Energy
and move the step EARLIER
DECREASE Activation Energy
and move the step EARLIER
3.  INCREASE contribution
of the current step
INCREASE contribution
of the current step
4.  DECREASE contribution
of the current step
DECREASE contribution
of the current step
5. ![]() INCREASE Activation Energy
and move the step LATER
INCREASE Activation Energy
and move the step LATER
6. ![]() MOVE current reaction step
to the LATER time
MOVE current reaction step
to the LATER time
7.  SPREAD current reaction step
SPREAD current reaction step
8.  COMPRESS current reaction step
COMPRESS current reaction step
9.  SHARPEN current reaction step
SHARPEN current reaction step
10.  FLATTEN current reaction step
FLATTEN current reaction step
4. General Figure and Curve Description Used In This Guide
In the all figures of this document:
-
the marker points are the experimental data,
-
the solid lines are the simulations for the selected reaction step.
5. Using of the Adjusting Arrow Buttons
The experiment is done three times for different heating rates and presented as three experimental curves in the X-temperature view.
5.1  Move the Step EARLIER by INCREASING Pre-Exponential Factor (Heating)
Move the Step EARLIER by INCREASING Pre-Exponential Factor (Heating)
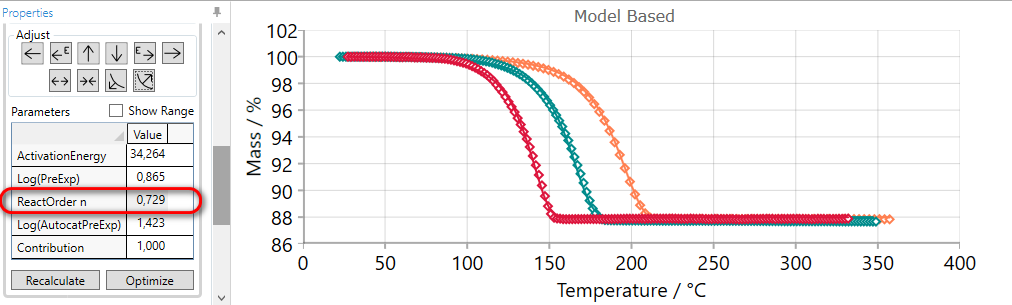
 Here the simulated solid curves for selected step show the mass change much later than the mass change in the experimental data.
In this case the simulated step can be moved to the earlier time (less temperature) by the using of the EARLIER arrow.
Here the simulated solid curves for selected step show the mass change much later than the mass change in the experimental data.
In this case the simulated step can be moved to the earlier time (less temperature) by the using of the EARLIER arrow.
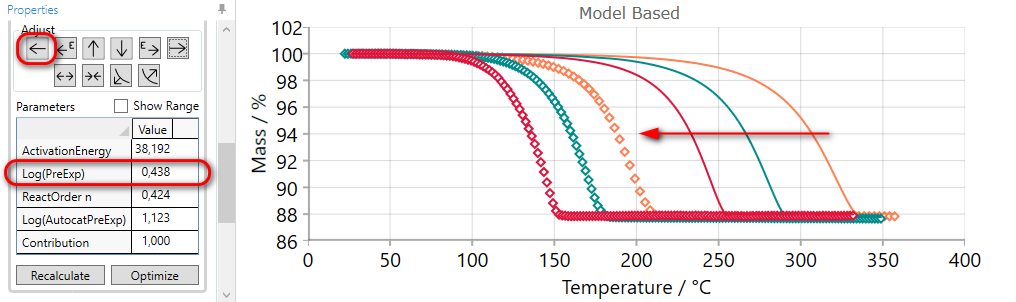
After clicking on the EARLIER arrow button  the pre-exponential factor increases and the step on simulated curves is moved to the left in direction of earlier time or less temperature
(for heating).
the pre-exponential factor increases and the step on simulated curves is moved to the left in direction of earlier time or less temperature
(for heating).
It may be necessary to click on the arrow button several times to “move” the simulated curves near enough to the measurements.
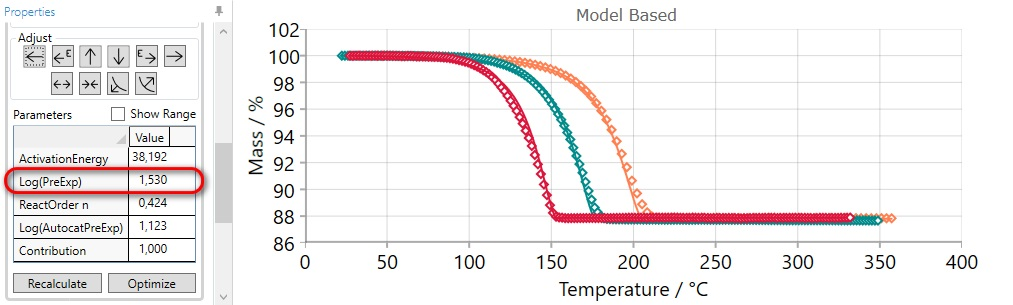
After manual adjustment please click on “Optimization” button. After that the result will be:
5.2  Move the Step EARLIER by DECREASING Activation Energy (Heating)
Move the Step EARLIER by DECREASING Activation Energy (Heating)
If the simulated curves for selected step are too late, then they can be moved to the earlier time by the using of DECREASE ACTIVATION ENERGY arrow. It is similar like in §6.1, but here the activation energy and not the pre-exponential factor will be decreased.

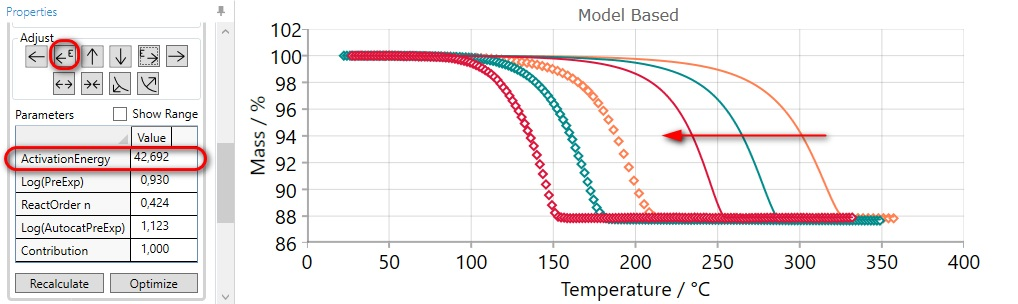
After clicking on the (DECREASE ACTIVATION ENERGY) arrow button  the value of activation energy decreases and that moves the
simulated curves to the earlier time or less temperature (for heating).
the value of activation energy decreases and that moves the
simulated curves to the earlier time or less temperature (for heating).
It may be necessary to click on the arrow button several times to “move” the simulated curves near enough to the measurements.
After manual adjustment please click on “Optimization” button. After that the result will be:
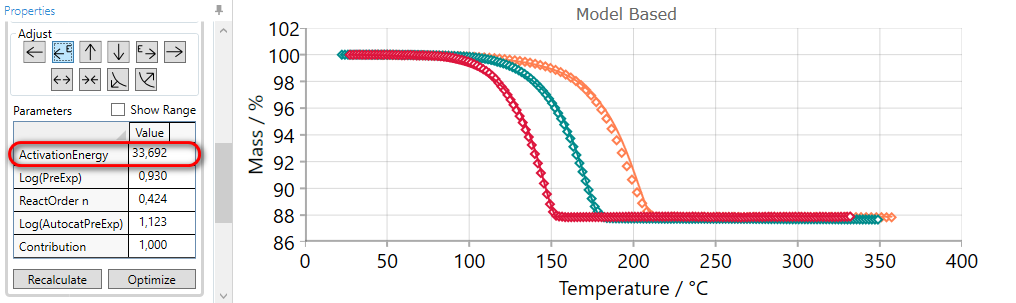
5.3  INCREASE Contribution of the Current Step
INCREASE Contribution of the Current Step
If the simulation for selected step finishes at too low conversion value in Conversion view, then the contribution of the current step can be increased by the using of INCREASE CONTRUBUTION arrow.
Here the final conversion for the first step is too low, therefore the first step should be selected.
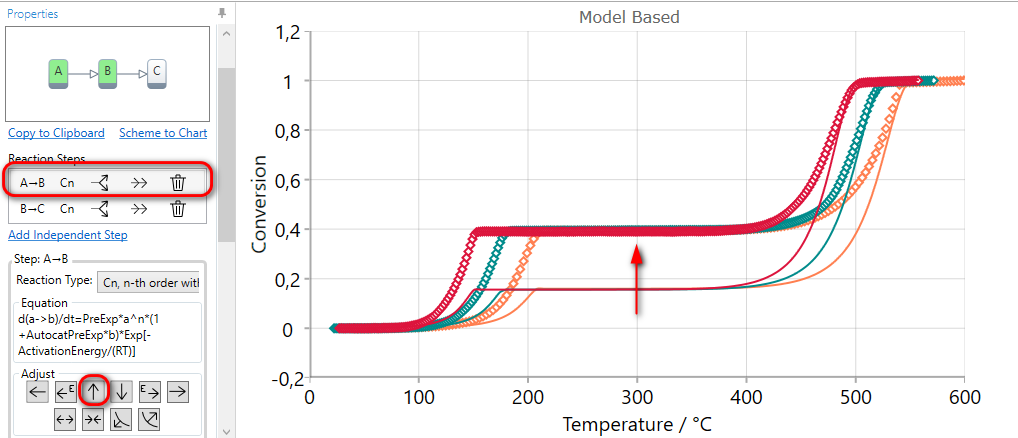
Using of INCREASE CONTRUBUTION arrow for the selected step increases the value of the contribution and then the simulated curves have the higher value of final conversion for this step. The contribution values of other reaction steps are automatically reduced in order to have the sum of all contributions equal to one.
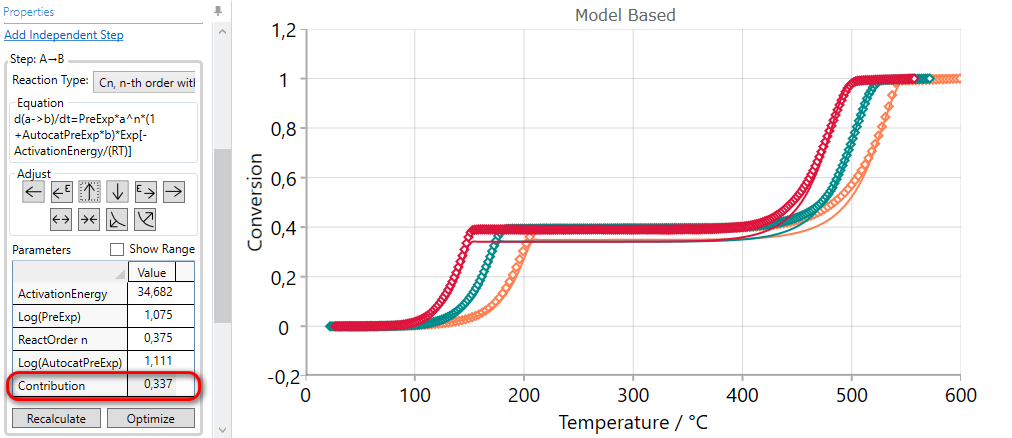
5.4  DECREASE Contribution of the Current Step
DECREASE Contribution of the Current Step
If the simulation for selected step finishes at too high conversion value in Conversion view, then the contribution of the current step can be decreased by the using of DECREASE CONTRUBUTION arrow.
Here the final conversion for the first step is too high, therefore the first step should be selected.
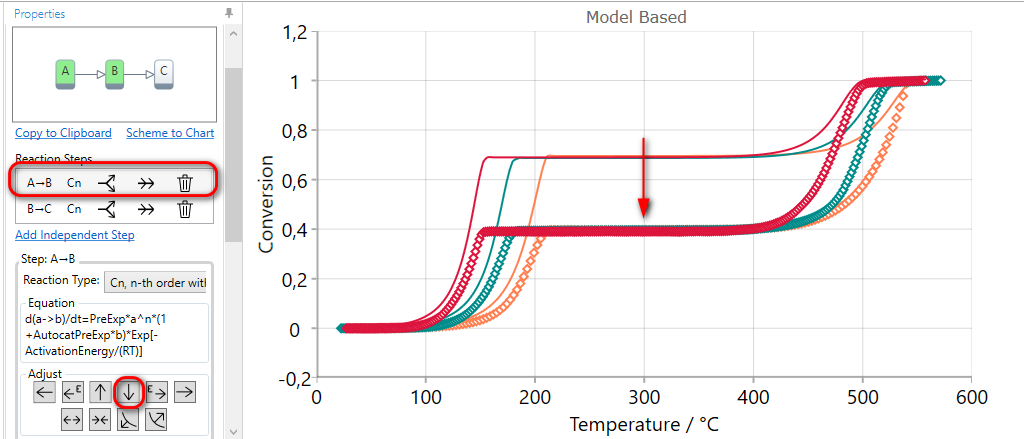
Using of DECREASE CONTRUBUTION arrow for the selected step decreases the value of the contribution and then the simulated curves have the lower value of final conversion for this step. The contribution values of other reaction steps are automatically increased in order to have the sum of all contributions equal to one.
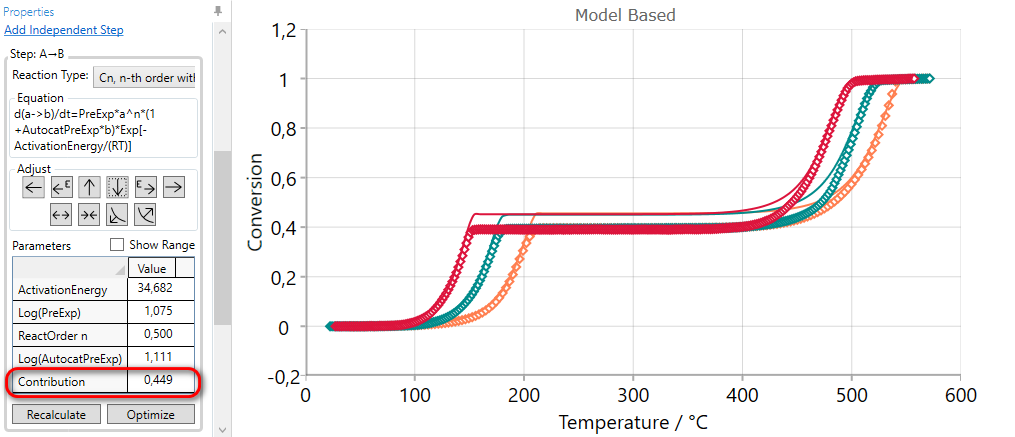
5.5  Move Reaction Step LATER by INCREASING Activation Energy
Move Reaction Step LATER by INCREASING Activation Energy
If the simulated curves for selected step are too earlier, then they can be moved to the later time by the using of INCREASE ACTIVATION ENERGY arrow.
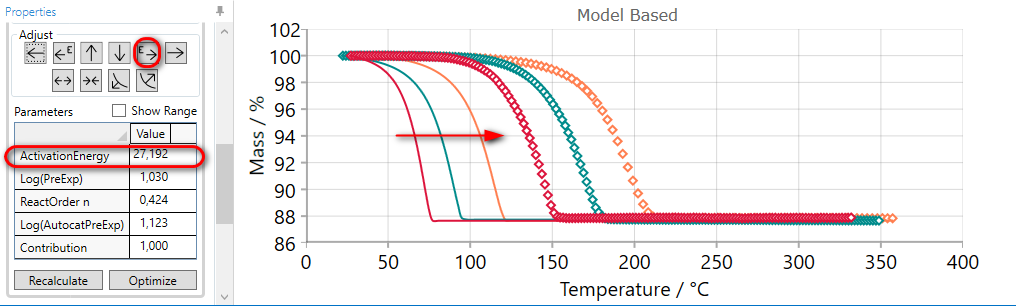
Using of INCREASE ACTIVATION ENERGY arrow increases the value of activation energy and moves simulated curves to the later time
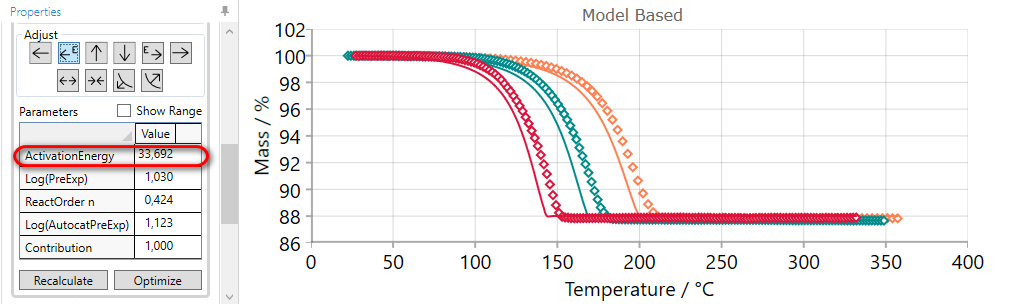
5.6  Move Reaction Step LATER by DECREASING Pre-Exponentian Factor
Move Reaction Step LATER by DECREASING Pre-Exponentian Factor
If the simulated curves for selected step are too early, then they can be moved to the later time by the using of LATER arrow.
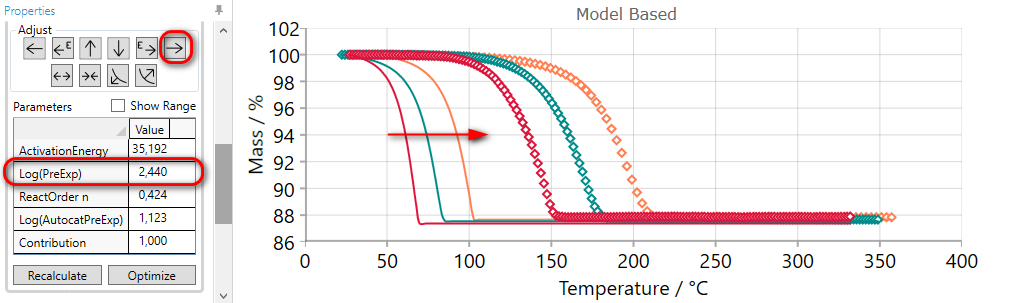
Using of LATER arrow decreases pre-exponential factor and moves simulated curves to the later time
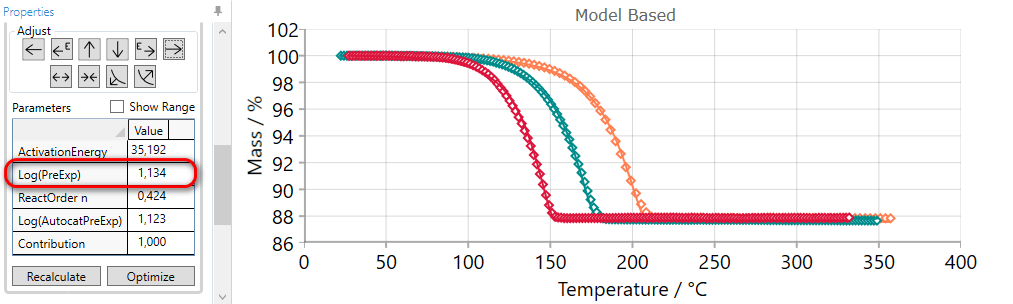
5.7  SPREAD Reaction Step
SPREAD Reaction Step
If the simulated curves for selected step are placed too close to each other, then they can be spread by the using of SPREAD arrow.
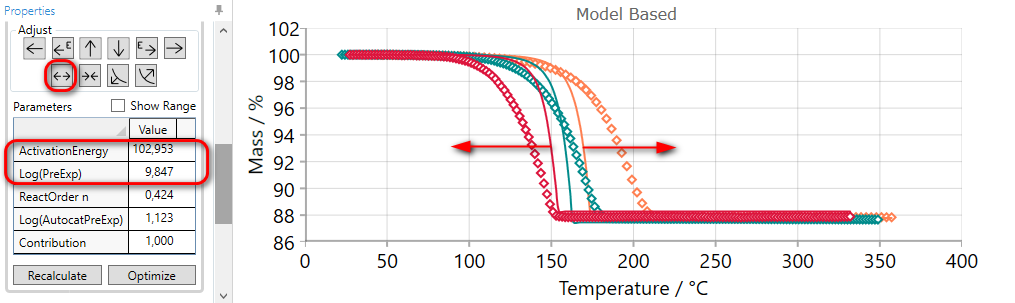
Using of SPREAD arrow decreases the value of activation energy and increases pre-exponential factor simultaneously. Thus, new simulated curves will be far from each other.
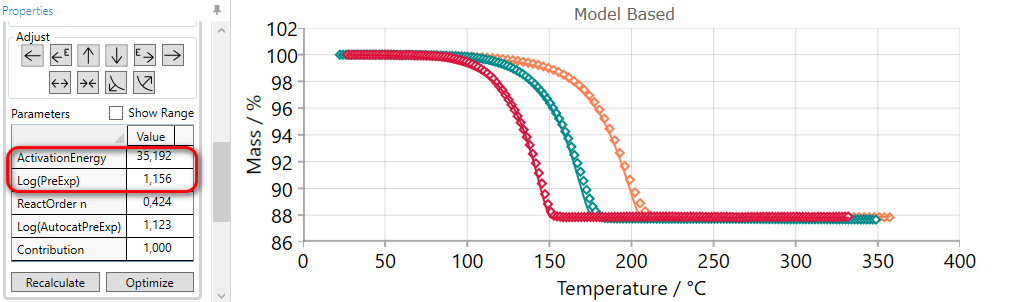
5.8  COMPRESS Reaction Step
COMPRESS Reaction Step
If the simulated curves for selected step are placed too far from each other, then they can be compressed by the using of COMPRESS arrow.
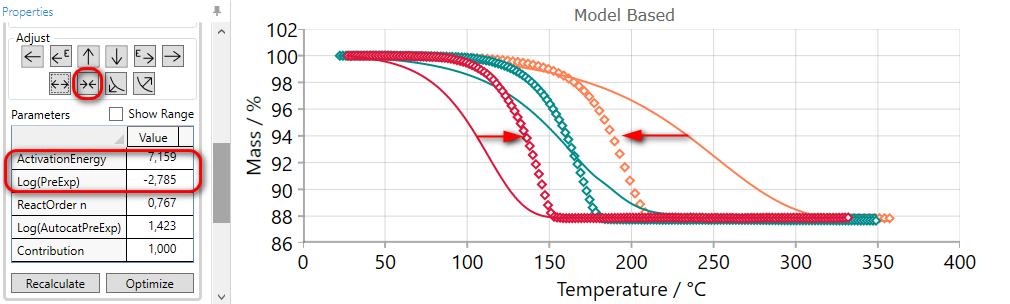
Using of COMPRESS arrow increases the value of activation energy and decreases pre-exponential factor simultaneously. Thus, new simulated curves will be close to each other.
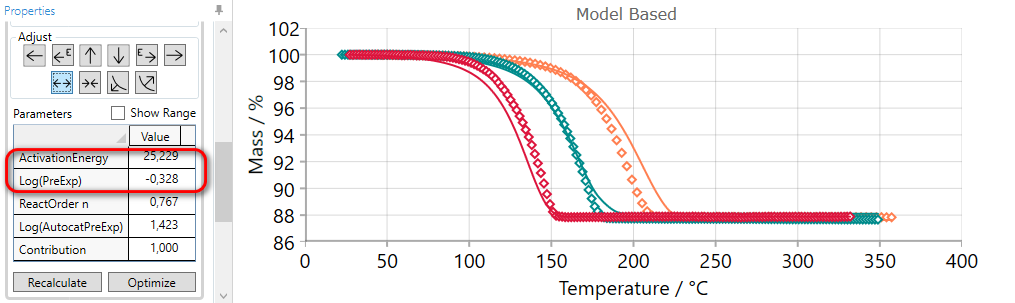
5.9  SHARPEN Reaction Step
SHARPEN Reaction Step
If the slope of the simulated curves for selected step is too low then they it can be increased by the using of SHARPEN arrow.
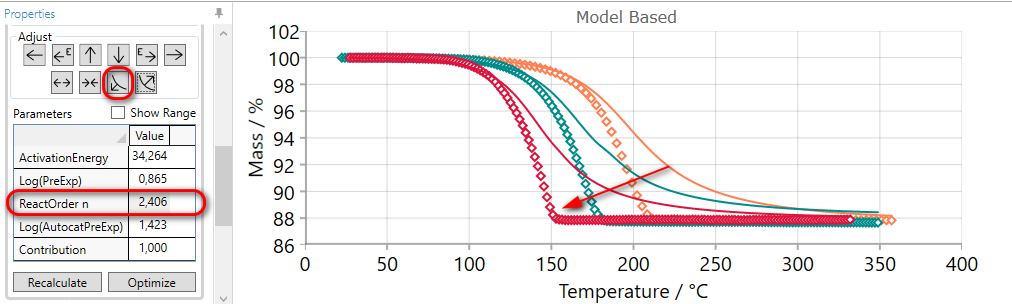
Using of SHARPEN arrow:
-
decreases the value of reaction order,
-
or increases dimension of nucleation for Avrami reactions,
-
or decreases autocatalytic order for reactions where reaction order is fixed.
This adjusting arrow gives no any changes for reaction types having no reaction order or nucleation dimension as the parameter.
5.10  FLATTEN Reaction Step
FLATTEN Reaction Step
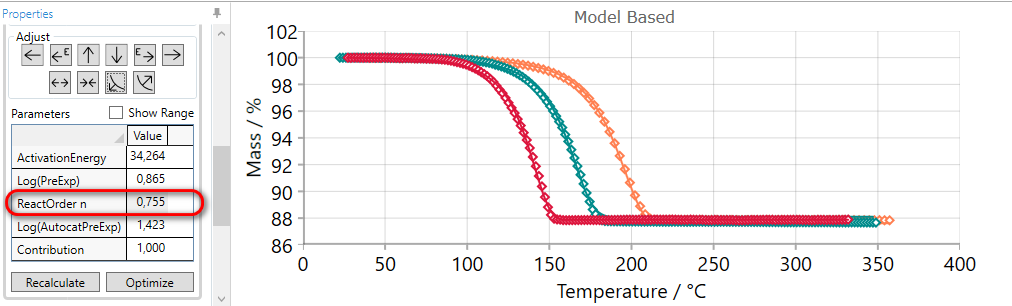
If the slope of the simulated curves for selected step is too high then they it can be decreased by the using of FLATTEN arrow.
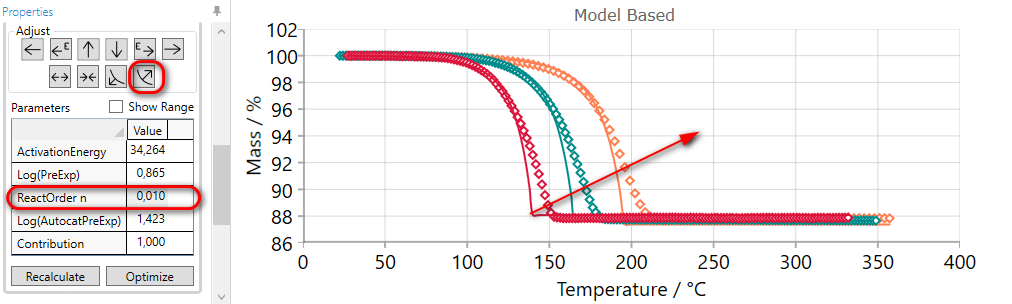
Using of FLATTEN arrow:
-
increases the value of reaction order,
-
or decreases dimension of nucleation for Avrami reactions,
-
or increases autocatalytic order for reactions where reaction order is fixed.
This adjusting arrow gives no any changes for reaction types having no reaction order or nucleation dimension as the parameter.